THE History of Eminent Spine

THE SWORD OF EMINENT
Created in the year 2020. A strong brand identity that is well recognized and easily identifiable. The sword of Eminent pays tribute to the world-class pedicle screw system.
Shaft: Wrapped with the triple lead pitch, which stands for “TRUTH, HONOR & PROFESSIONALISM”. The 3 core values of Eminent Spine.
Blades: Forged on solid biomechanical engineering principles and the best-known biomaterials known to man. These elements melted together results in the creation of unparalleled spinal implants.
Shield: Protects the principal values of Eminent Spine.
The logo is a symbol of strength and honorable values.
It is the logo of Eminent Spine.
Timeline of Eminent Spine
The evolution of Eminent Spine has always been focused on biomaterials and spinal implants for spinal surgeries. Since the introduction of the first PEEK interbody spinal fusion system to the advancement of the MIS pedicle screw system to the support of revolutionary 3D titanium printing technology, Eminent Spine has emerged as an industry leader in the evolution of spinal implants.
Below is a timeline of biomechanics and biomaterials, to create the most advanced spinal implants in the world.
2008
US-FDA 510(K) CLEARANCE FOR THE PEEK INTERBODY FUSION SYSTEM.
2009

US-FDA 510(K) CLEARANCE FOR THE PEEK INTERBODY FUSION SYSTEM.


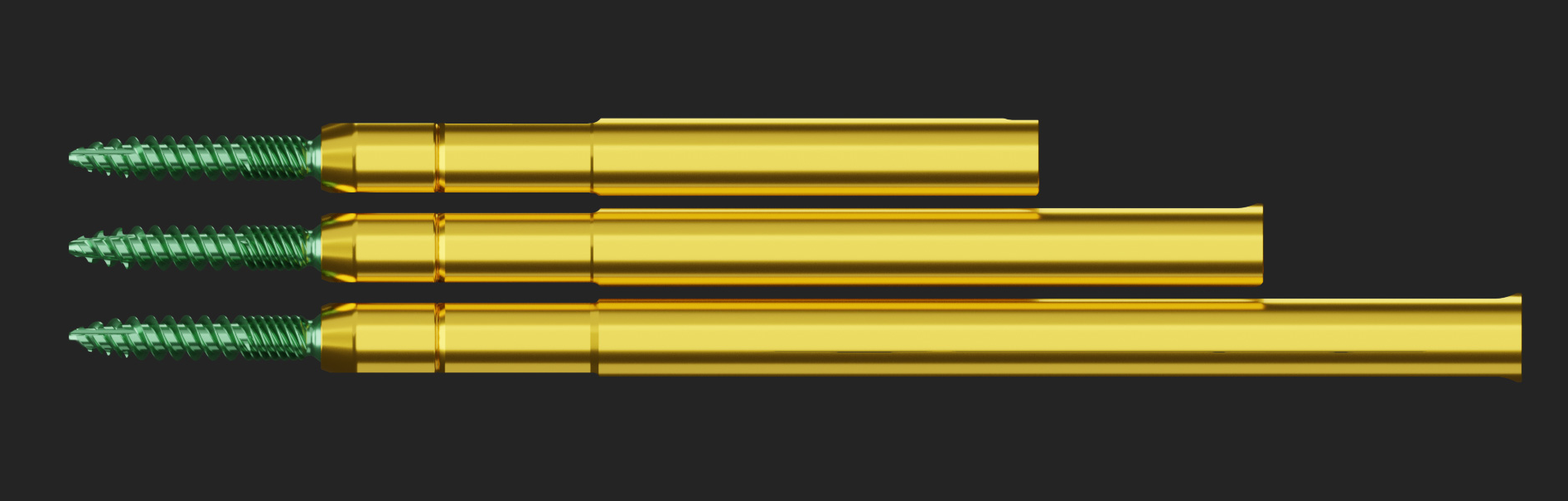
US-FDA 510(K) CLEARANCE FOR THE ANTERIOR LUMBAR BUTTRESS SYSTEM.
2010

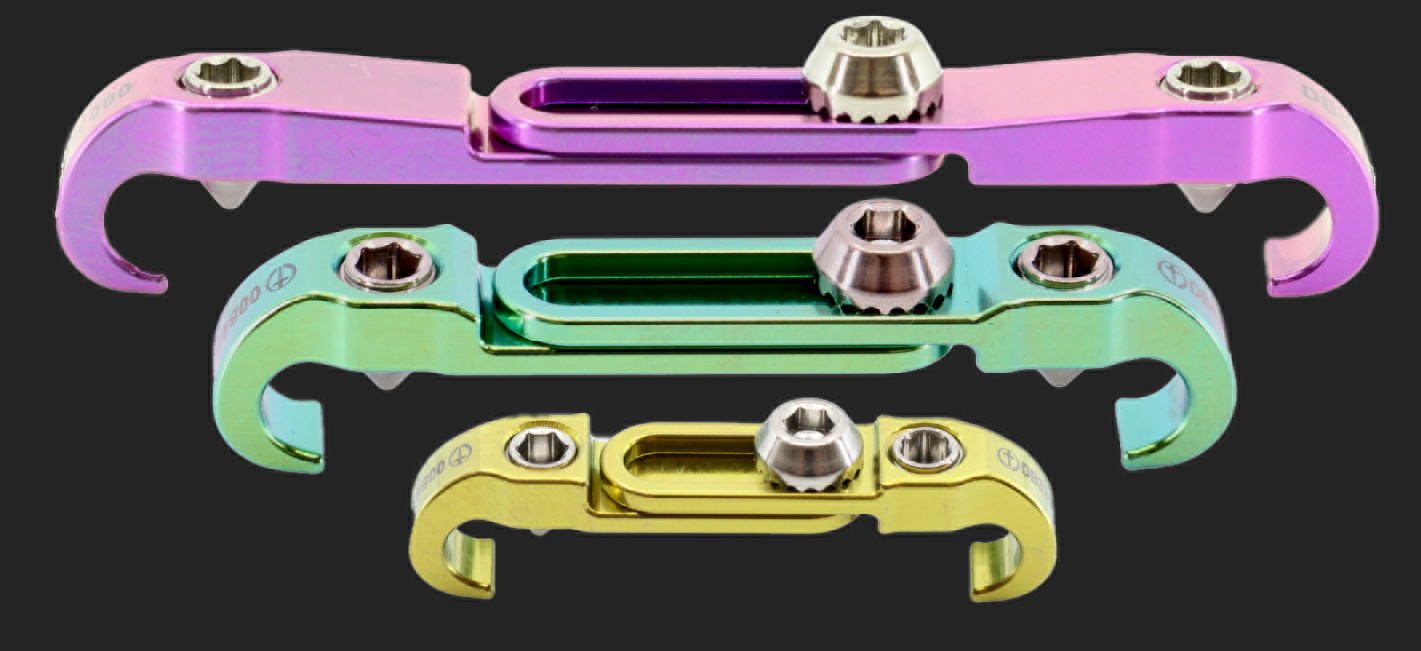
US-FDA 510(K) CLEARANCE FOR THE CANNULATED & NON-CANNULATED PEDICLE SCREW SYSTEM & THE LUMBAR CROSS-LINK.
2011
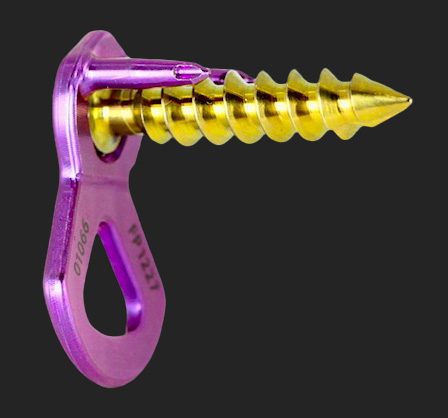
US-FDA 510(K) CLEARANCE FOR THE ANTERIOR CERVICAL PLATING SYSTEM.
EMINENT SPINE RELEASES THE ANTERIOR CERVICAL PLATE CLINICAL DATA.
EMINENT SPINE RELEASES THE LUMBAR PEDICLE SCREW & CROSS-LINK CLINICAL DATA.
2012
INTERNATIONAL SPINAL SALES BEGINS.
2013
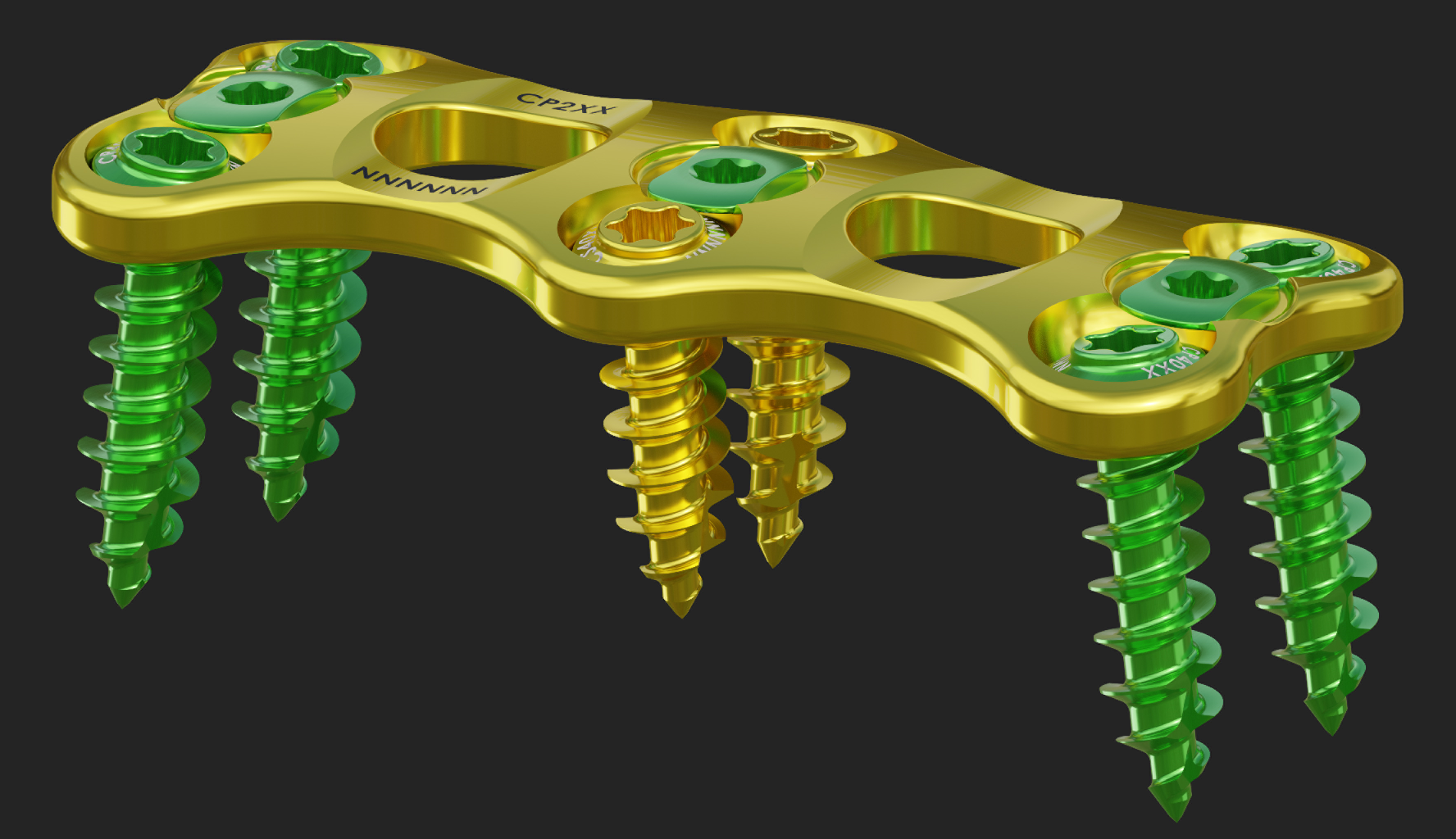
Pedicle MIS System released.

lumbar peek lateral cage system released.
2014
US-FDA 510(K) CLEARANCE FOR THE ANTERIOR LUMBAR PLATING SYSTEM.
2015
US-FDA 510(K) CLEARANCE FOR EMINENT EXTREMITIES.
FEBRUARY 2020

EMINENT SPINE UNDERWENT FULL TRANSFORMATION INCLUDING REBRANDING, ADMINISTRATION CHANGES AND NEW FDA CLEARANCES.
RELOCATED TO NEW HEADQUARTERS IN PLANO, TX.
JUNE 2020
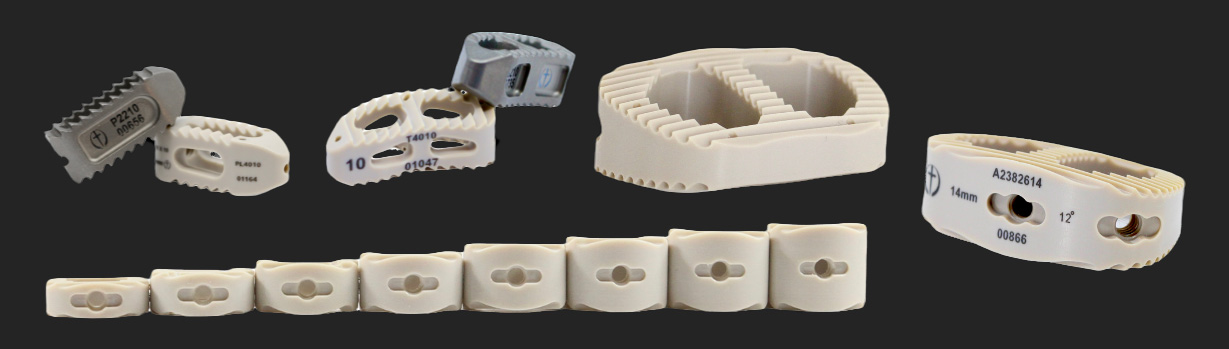
LUMBAR AND CERVICAL PEEK IBFD SYSTEM UPDATED.
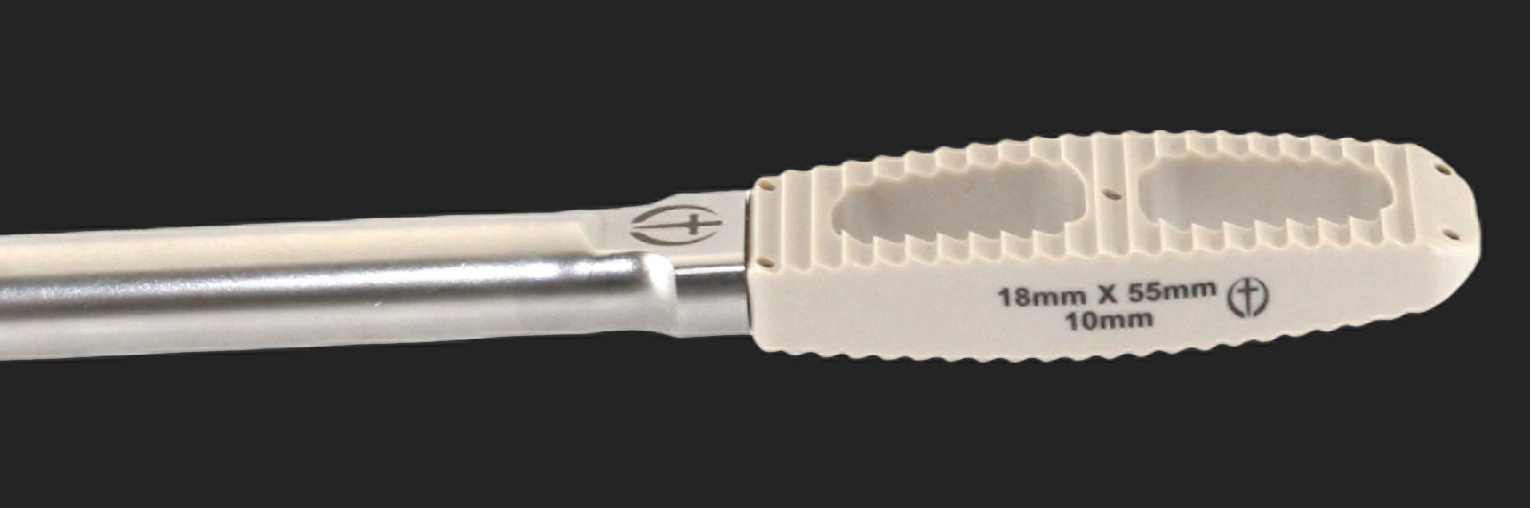
LUMBAR PEEK LATERAL IBFD SYSTEM UPDATED.
OCTOBER 2020

US-FDA 510(K) CLEARANCE FOR THE MODERN, LOW-PROFILE ANTERIOR CERVical plate system.
NOVEMBER 2020

rELEASE OF C3 & s3 PEDICLE SCREW (TAPERED TIP & tHICKER NECK).
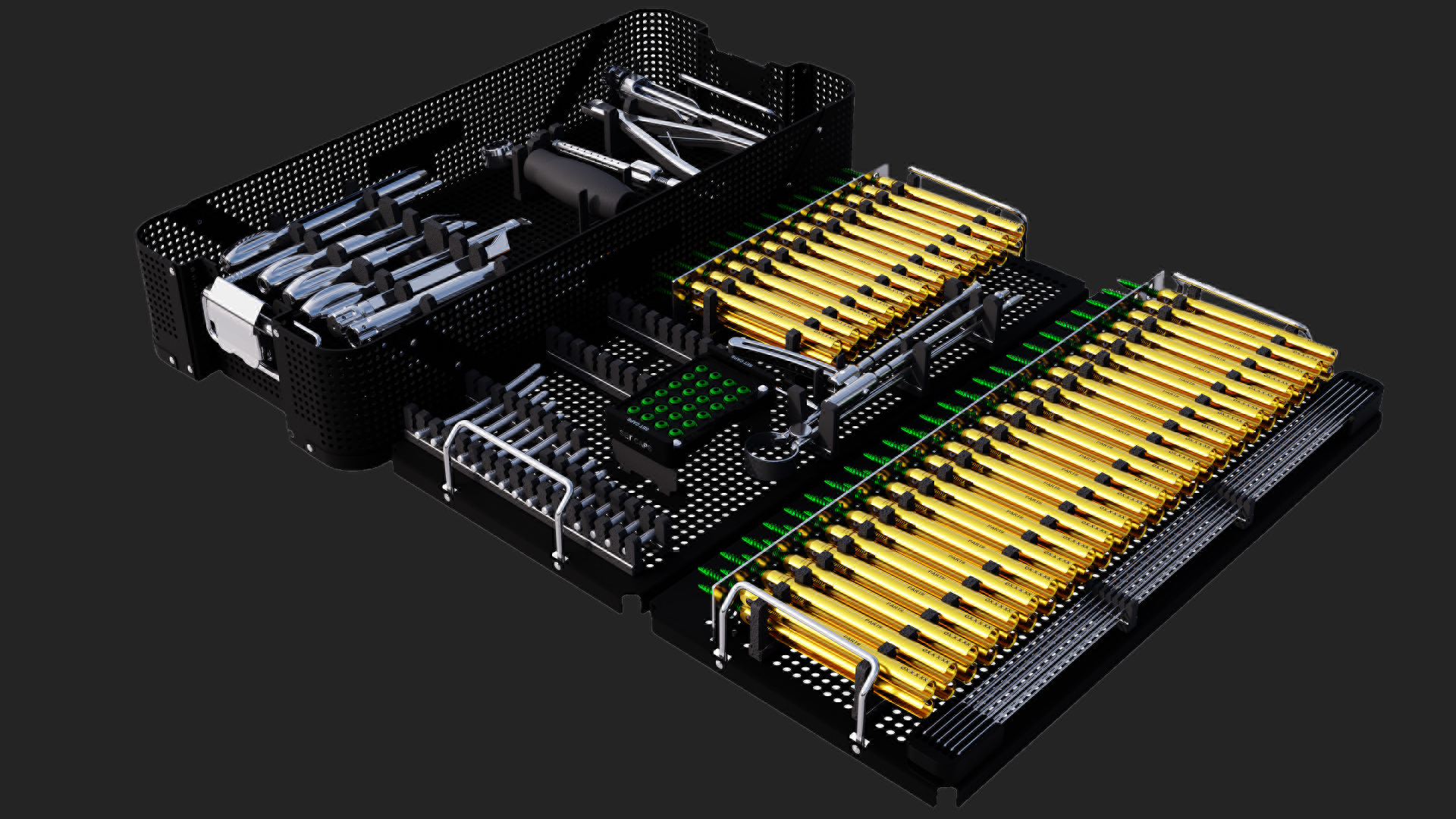
Release of 4.00MM MIS Pedicle Screw Towers & INSTRUMENTATION DELIVERY SYSTEM.
Release of Grit Blasted Pedicle Rod with improved Lordosis & Marking.
JANUARY 2021
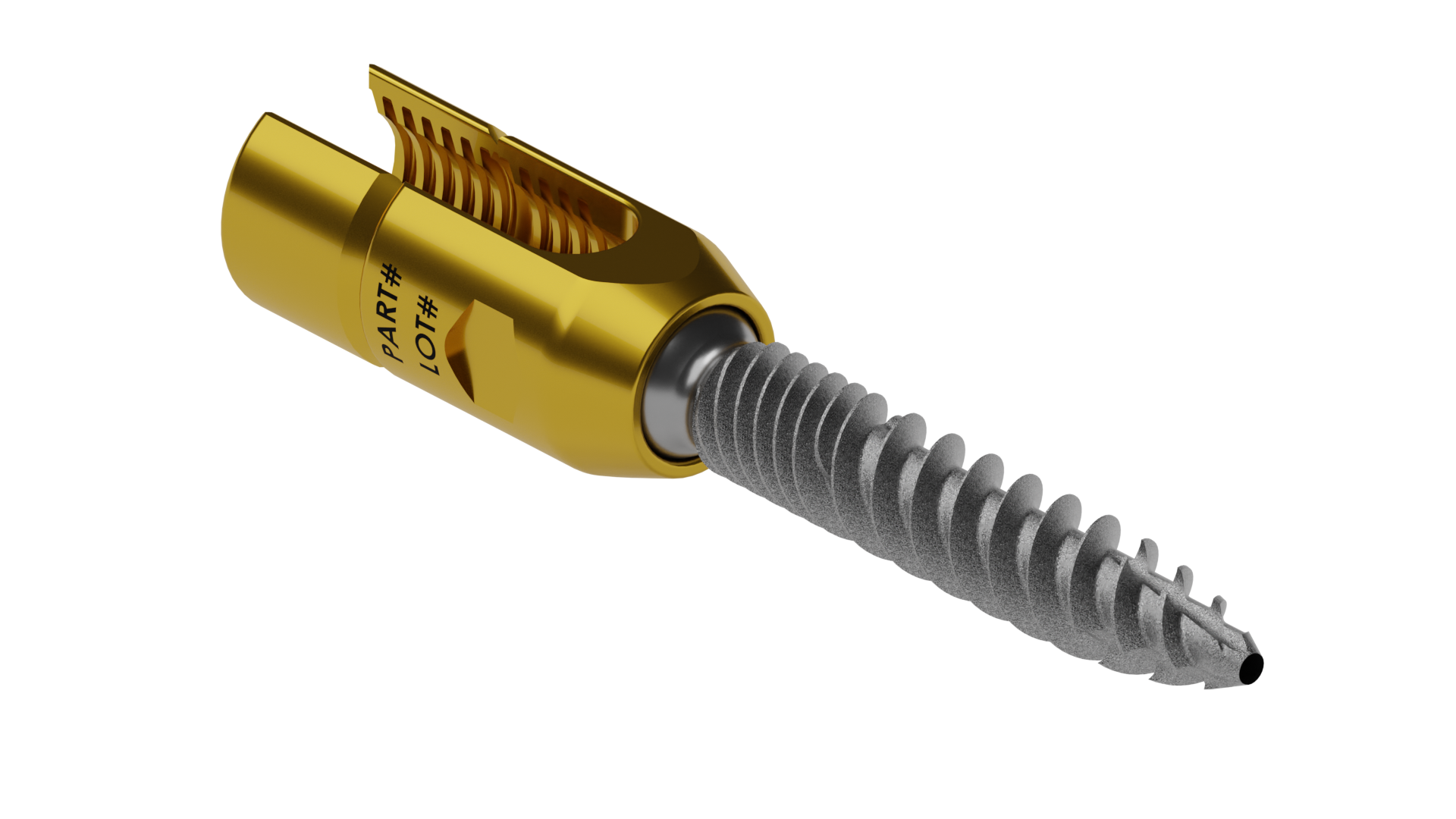
RELEASE OF SURFACE TEXTURE PEDICLE SCREW.
RELEASED 4.75MM CANNULATED PEDICLE SCREW.
NOVEMBER 2021










EMINENT SPINE’S CERVICAL STAND-ALONE SYSTEM INCLUDES NON-STERILE IMPLANTS WITH A WIDE RANGE OF IMPLANT PROFILES WITH BOTH FIXED AND VARIABLE SCREW OPTIONS.
CERVICAL PEEK, TITANIUM & 3D TI CAGES ARE OFFERED IN OUR STAND-ALONE SYSTEM.
US-FDA 510(K) CLEARANCE AS OF NOVEMBER 5, 2021.
OCTOBER 2022

EMINENT SPINE’S LUMBAR STAND-ALONE SYSTEM INCLUDES NON-STERILE IMPLANTS WITH 5 IMPLANT PROFILES AND hyperlordotic angles.
lumbar PEEK & 3D TI CAGES ARE available IN OUR STAND-ALONE SYSTEM.
US-FDA 510(K) CLEARANCE AS OF october 17, 2022.
FEBRUARY 2023

Eminent Spine’s Cervical 3D Titanium cages are OFFERED non-sterile.
US-FDA 510(k) Clearance AS OF February 6, 2023.
MAY 2023
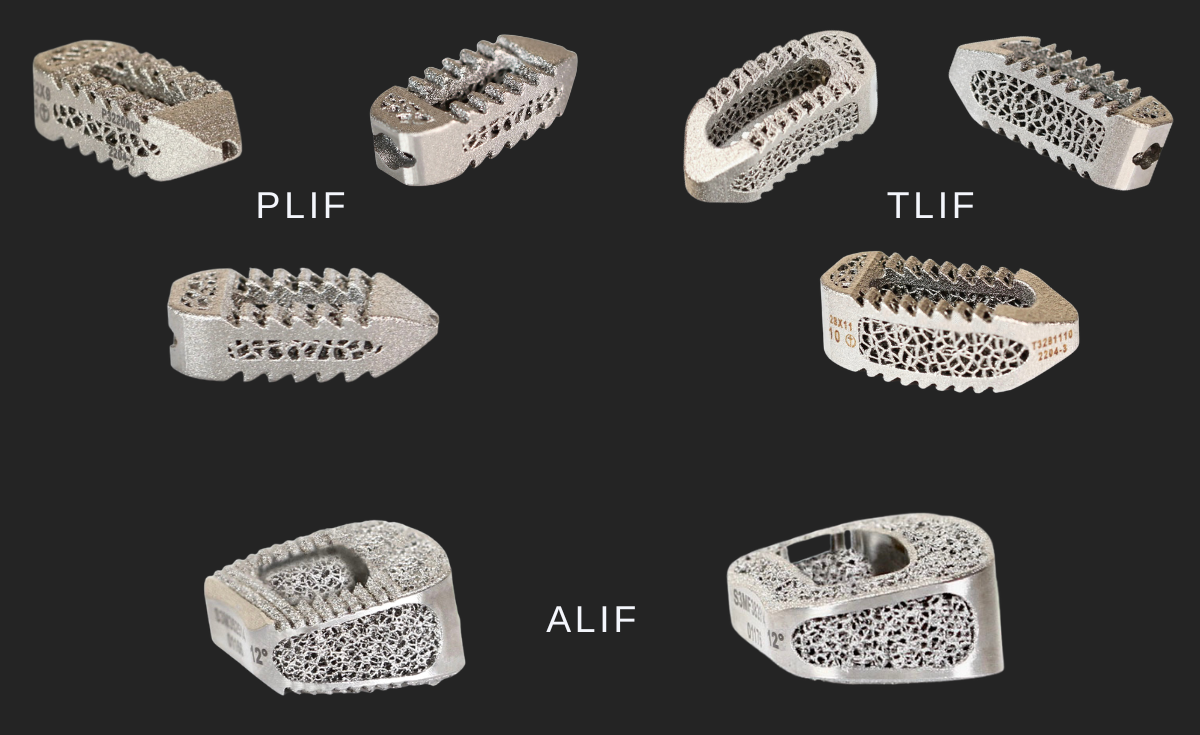
Eminent Spine’s 3D Titanium lumbar interbody fusion systems: PLIF/TLIF/ALIF.
US-FDA 510(k) Clearance AS OF may 15, 2023.

